Early prototype design of the KLARO™ deep cavity surgical LED lighting device
KLARO™ is a next-generation surgical LED lighting device specially developed for deep-cavity open surgery.
During open surgery, overhead lights in the operating theatre currently result in difficulties obtaining proper visibility especially during open deep cavity procedures. This is primarily caused by shadows cast by surgical personnel blocking the overhead lighting whilst bending over the surgical cavity as well as the physical angle/slanted nature of the narrow surgical opening which makes it difficult for external light to enter.
The device is currently target for general open surgery purposes and is easy to use, providing localised, uniform and high intensity “flood-lighting” from within the surgical site. Its small diameter (less than 4.8 mm) means it can fit unconstructively in-between organs or within narrow internal cavities, allowing the surgeon to work with maximum visibility and excellent illumination.
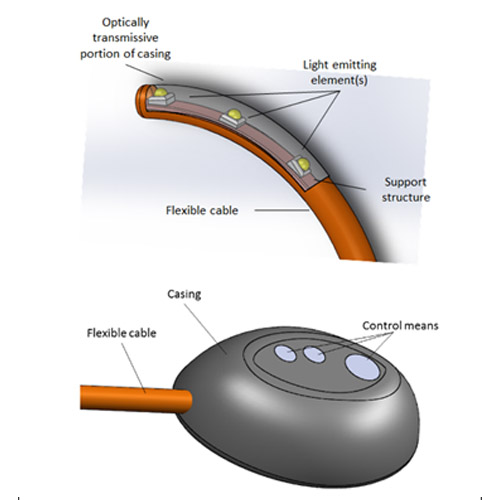
The KLARO™ deep cavity surgical LED lighting device comprises the following key features:
- Sterile and single-use battery-operated disposable
- Discreet form factor allows device to fit unobtrusively within any open surgical cavity
- Safe working temperature of < 38°C maintained throughout operating lifespan
- Wide and flexible illumination angle of over 180°
- Operator adjustable illumination intensities (4 settings)
- Self-retaining i.e. the user is not required to hold onto the device during surgery
One of the device’s key hallmarks is its ability to maintain a safe and cool working temperature (< 38°C) throughout use, which is well-matched for applications inside of the human body, regardless of the luminous intensity selected by the operator.
Besides generously supporting the KLARO™ project with its Innovation to Develop (I2D) grant scheme, NHIC was instrumental in acting as the bridge between Vivo Diagnostics and SingHealth’s R&D teams from the National Cancer Centre (NCC) and Medical Technology Office (MTO). This helped very much to facilitate contractual and licensing negotiations between the parties and allowed SingHealth’s teams to better understand and appreciate the commercial requirements and expectations of the project from Vivo’s perspective.
Additionally, NHIC provided the project with support and advice from a quality management and regulatory angle, which greatly helped in the planning of the product’s route to market.
At present, KLARO™ has successfully completed clinical trials and is undergoing final engineering steps by us and our co-development partners in Europe and Singapore before the final version is submitted for testing and subsequent CE Mark and FDA registration.
The product will be ready for commercial sales by Q1 2019 and are now in the process of appointing distributors in several territories across Asia, North America and the EU.
For more information on business or investment opportunities, please email: enquiry@nhic.cris.sg

