Innovating Health: Securing Singapore’s public healthcare innovations
The journey from healthcare innovation to commercialisation is multi-faceted, requiring collaboration, strategic planning and partnerships, along with robust IP protection and strategies. IP rights, patents, and licensing are essential components of an effective IP strategy, which allows the protection and scaling of innovative ideas with the potential for wide-reaching impact.
This World Intellectual Property Day, we shine a spotlight on how the National Health Innovation Centre (NHIC) partners with the three public healthcare clusters to assist clinician innovators in protecting their original ideas and maintain a competitive edge. These include:
-
The National Healthcare Group’s (NHG) Centre for Medical Technologies and Innovations (CMTi)
-
National University Health System (NUHS) Research Office
Each of these offices support their clinical innovators in developing their novel ideas into solutions which address unmet healthcare needs, and in test-bedding these solutions in real-world healthcare settings. Additionally, they manage a vast range of responsibilities from guiding, protecting, commercialising inventions, and serving as a one-stop access for innovators within their clusters.
In our conversations with representatives from each healthcare cluster, they share insights into the importance of protecting the IPs, success stories, and the ongoing collaborative efforts with NHIC to foster an ecosystem conducive to healthcare innovation.
Enabling collaborative innovation together with NHIC
NHIC supports clinical innovations from ideation through development to commercialisation and adoption, powering the next generation of healthcare innovation. In particular, the Innovation to Protect (I2P) support scheme provides funding to fund patent expenses for patentable inventions with demonstrated proof of concepts.
To further enhance the innovation and enterprise ecosystem, NHIC also supports each cluster through events, consultancy services, workshops and stakeholder engagement to ensure that clinical innovators receive the necessary support to navigate the process of commercialisation and market access including applying for IP rights effectively.

Dr Alfred Chia, Senior Assistant Director (left) and Zhang Bo, Senior Executive (right) at NUHS Research Office provide support to researchers and investigators across NUHS at every step of the innovation journey to help them stay at the forefront of innovation in healthcare.
Highlighting the importance of these outreach efforts, Dr Alfred Chia, Senior Assistant Director at NUHS Research Office notes the critical role of understanding the landscape, including prior art, to enhance the success of patent applications. These initiatives aim to benefit not just NUHS but the broader public healthcare innovation ecosystem.
“Often, innovators file for patents based on what they think is inventive and novel, however, they do not consider the competitors or what we call “prior art” (which refers to any invention which has been made available to the public anywhere in the world by written or oral disclosure before the Principal Investigator’s (PI) patent application is filed),” he said.
Zhang Bo, Senior Executive at the Innovation Transfer Office at the NUHS Research Office shared that navigating the intricacies of innovation also involves tough decisions such as saying “no”. Despite clinicians' initial excitement about their ideas, Zhang Bo added that at times, he had to turn down concepts that may not be feasible due to the lack of inventiveness or because the idea did not demonstrate significant improvement on prior art.
Dr Erin Teo, Assistant Director at SHIP added: “In order for a patent application to secure a strong patent position, it should be filed before the researcher publishes or publicly discloses their invention. As such, education of researchers is of pivotal importance.”

Dr Erin Teo, Assistant Director (left) and Chole Chu, Assistant Manager (right) at SHIP guide clinicians and researchers towards protecting their inventions and moving it along commercialisation pathways that will improve the lives of patients and deliver better healthcare.
The dedication of these officers at the healthcare clusters ensure that ground-breaking ideas are not only protected but also supported from early-on, so that it can develop further and be brought to market eventually.
At the core of these endeavours, the teams from each of these clusters also encourage open dialogue and collaboration to streamline the innovation process within their respective clusters.
NHG CMTi, for example, has the mandate to drive the end-to-end Innovation and Enterprise (I&E) ecosystem in NHG.
They collaborate with research institutions to identify research outcomes that can be translated into real-world applications, as well as support the development of innovative ideas, facilitate clinical and validation trials, and coordinate their implementation and adoption. Additionally, CMTi provides NHG PIs access to venture building networks, where budding start-ups and spin-offs are formed and supported, contributing to a conducive ecosystem for innovation.
In this way, the NHG CMTi I&E ecosystem is well-oiled to oversee IP management, cross-institution collaborations, and drive the development of clinicians’ ideas towards tangible products with an eye for commercialisation.

Hannah Chong, Assistant Manager (left) and Evangeline Ng, Manager (right) at CMTi support NHG’s clinician innovators to transform good ideas into an eventual product.
“Over the years, the innovation culture in Singapore has shifted to steer the end point focus of innovation and enterprise beyond development towards adoption and commercialisation. Centralising IP management at NHGHQ level, gives us an overview of the IP assets. This allows the team to ensure timely and strategic management of these Intangible Assets.” said Evangeline Ng, Manager at CMTi, NHG Group Research and Innovation.
“At CMTi, we play a deep seeding role at the developmental stage, guiding clinicians to mine for unmet clinical needs and match them with competent partners to translate these into products. Through the process, we work with partners like NHIC, National Medical Research Council (NMRC) and Singapore Biodesign to groom clinician innovators through national-level funding and talent development programmes. Eventually, we facilitate the adoption and commercialisation of new technologies, through coordination of test bedding, trials and new venture creation,” said Hannah Chong, Assistant Manager at CMTi, NHG Group Research and Innovation.
“We appreciate NHIC’s advice. They guide us towards developing innovations in the deep tech space. This proactive approach not only increases our chances of securing the right grants but also enhances the quality of research and innovation within our ecosystem,” added Hannah.
“Another primary challenge faced by institutions in the healthcare sector is that many innovations are still in early-stage, and hence to reach the technology transfer stage would take a relatively longer runway,” said Chole Chu, Assistant Manager at SHIP.
To alleviate some of these challenges, NHIC jointly collaborates with each of the public healthcare clusters in setting up Joint MedTech Grant (JMT) to support and propel the technology readiness level of early-stage innovations.
Both Evangeline and Hannah shared that the synergy between CMTi and NHIC has enabled NHG PIs to strengthen project proposals, gain deeper insights into market analysis and better assess the suitability of their proposals when applying for different grant schemes.
Recent milestones for the CMTi team include supporting more than 300 clinician innovators to-date and managing more than 380 active innovation projects. In the past year, they have completed 23 IP filings and 24 IP disclosures.
Evangeline cited a significant project which NHIC had supported since the early stage of development. The project received seed funding through the NHG CMTi-NHIC Joint MedTech Grant (NHG CMTi-NHIC JMT) alongside NHG funding, to develop an AI-powered algorithm software and dermoscope that is universal, affordable and attachable to analyse skin images from consumer-grade cameras. The software provides a fast and accurate method to detect high risk or malignant skin lesions. It eliminates the need for bulky or multiple devices to capture and transfer skin images for analysis, allowing for prompt follow-up care for patients.
Evangeline also highlighted an ongoing project where the PI is developing a first-of-its-kind non-invasive device that uses magnets and blood flow sensing technology to assess patients’ blood vessels for signs of inflammation and detect potential vascular complications in people with diabetes.
With NHIC’s support, the patent was filed together with a local polytechnic. The project has also garnered funding support from NHIC’s Innovation to Develop (I2D) grant and progressed to receive the Innovation to Industry (I2I) grant support where it continues to be developed.
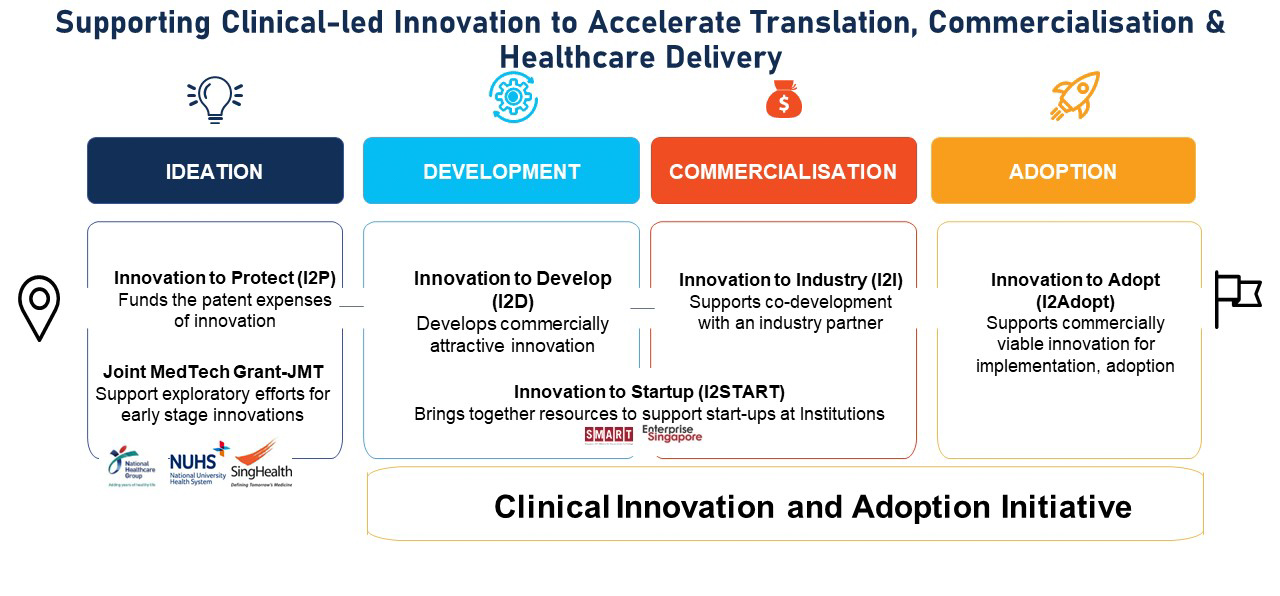
NHIC supports clinical innovations from ideation through development to commercialisation and adoption through various grant schemes.
“NHIC’s I2P funding scheme has enabled the filing of this IP which was supported through its I2D and I2I programmes. In collaboration with our partners, we are excited to see the continued development of this product. We hope it will move towards commercialisation one day,” said Evangeline.
Advancing market access for healthcare innovations
For healthcare innovations looking to enter the market, navigating market fragmentation due to differences in the healthcare systems, workflows, country-specific regulations, and clinical needs are some key considerations. Innovators also need to factor in ensuring sufficient IP protection and thorough survey of competitive market environments.
To overcome these hurdles, a deep understanding of the selected market, a robust network of collaborators, coupled with a strong strategy are essential for success.
Alfred from NUHS shared a recent success story which involved a portable user-guided visual acuity test developed by a PI, that allows patients to independently measure their eyes’ ability to distinguish shapes and colours. After a meticulous three-year prototyping process, the PI was looking for a commercialisation partner and several companies were eager to collaborate.
This project was memorable as the team had to overcome the challenge of simultaneously submitting regulatory documents and securing licensing terms for the device. Together with NHIC, the NUHS Research Office also provided guidance to the commercial partner on win-win ways to carry out the technology transfer process with the PI.
“Thanks to support from our partners, including NHIC, we achieved regulatory approval from the Health Sciences Authority in less than three months – a remarkable feat – from prototype to market,” said Alfred.
In the roadmap ahead, this invention will be a key component of a suite of community-based vision screening tools that will leverage AI to detect disease-related visual impairment and measure intraocular pressure for glaucoma monitoring. Alfred shared that the team’s next focus is to identify distribution opportunities for this device across Asia.
“NHIC has been a very important partner to the NUHS Research Office as we evaluate the market potential of ideas together,” said Alfred. “When PIs and researchers are successful in their grant applications for their IP, it motivates other innovators to get their IP applications backed by a national programme office such as NHIC,” he added.
Hannah from NHG also highlighted a memorable innovation from the National Skin Centre (NSC), which developed a clinical-grade pain alleviation device that provides vibrational stimulus to relief discomfort on the skin and offer pain management during dermatological procedures such as cryotherapy.
This pain alleviation device addresses a gap in the clinical setting, and presentations about its technology and how it works has been well received by dermatologists at both local and international conferences.
In the early days, this innovation received funding support from the NHG CMTi-NHIC JMT grant. Along the development pathway, this device was also licensed to an industry partner for further commercialisation.
“We are also thankful for opportunities to be invited by NHIC to co-exhibit at trade events. Interestingly, at the Singapore Week of Innovation and Technology (SWITCH) 2022, the device was showcased within NHIC's booth and this innovation's commercialisation partner received its first overseas order at the event,” said Hannah.
Striking a balance to optimise resources
Chole from SingHealth shared that the path of bringing an idea from bench to bedside requires a hardy resolve to overcome hurdles and strike a delicate balance of allocating available resources.
“Hence, our team would have to evaluate new inventions while managing the existing IP portfolio,” said Chole.
Erin recalled a memorable project supported by various NHIC grants to support the technology transfer and commercialise a device to treat patients with a condition that causes heart valve leaks or “leaky heart”.
“It was a technical medical device with many aspects – from how it was engineered, to its deployment. Together with the NHIC team, we advised the PI on the various patents that may be applicable to this device,” said Erin.
She illustrated that the idea was to have “a very comprehensive and strong patent “army” (sufficient patents to cover various aspects of the innovation (i.e. core technology, methods, design) as protection against competitors and intellectual property infringement) that would allow the device to be licensed into a start-up, and hence it was crucial to “ensure all patentable aspects were covered.”
“NHIC's ongoing support, particularly through the I2P scheme, has been instrumental in enhancing SHIP’s ability to protect and manage its intellectual property assets,” she added.
Forging future collaborations for the road ahead
Looking ahead, the representatives from all three clusters note that there are ample opportunities for deeper collaboration with NHIC for the road ahead.
These include leveraging shared resources and expertise in regulatory guidance, business strategy, and technical development for capability development of the innovators within the clusters.
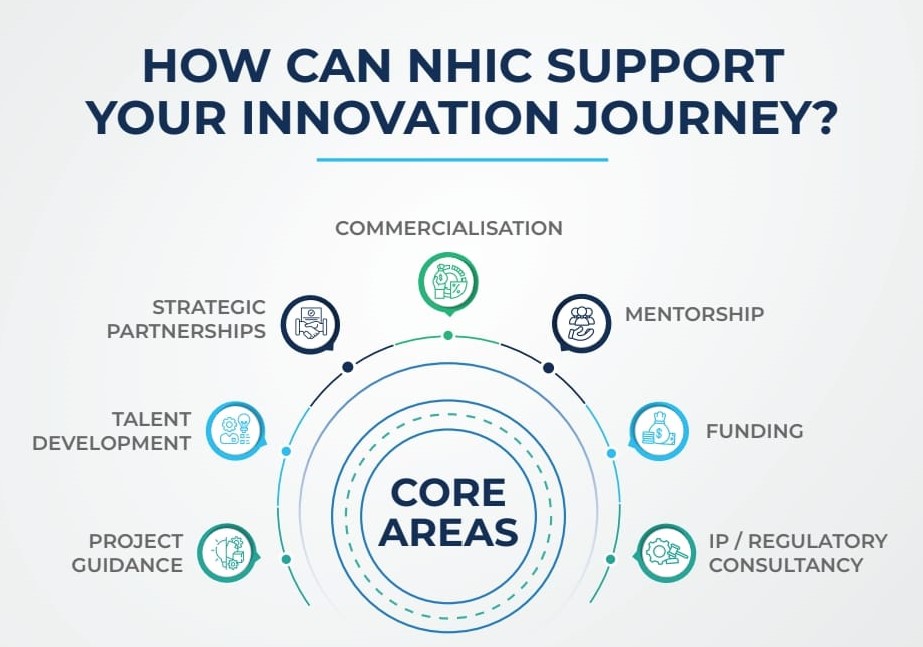
NHIC funds the translation and development of medical innovations towards a market-ready product. Beyond funding, NHIC provides strategic guidance on regulatory information, business development and other business activities, as needed by the individual projects, thus accelerating the pathway to impact healthcare.
NHIC has also been working with c011ab Novena – a tripartite alliance between Nanyang Technological University, Singapore (NTU Singapore), Agency for Science, Technology, and Research (A*STAR), and the National Healthcare Group (NHG) – to advise budding companies and start-ups on how to track their development roadmap, draw up targets towards significant project milestones and offer advice on industry partners they can connect with on their innovation journey.
There are also plans to conduct a workshop on IP portfolio and strategies in partnership with NUHS in the coming year that will be open for all clinical innovators from public healthcare clusters / academic medical schools / healthtech institutes. These partnerships ensure clinical innovators receive tailored guidance and access to appropriate resources.
The healthcare innovation journey requires teams to adeptly navigate the dynamic intersection where healthcare intersects with science, business, and innovation. Further support such as these will strengthen partnerships and drive impactful innovation in healthcare.
For more information on how to collaborate with NHIC or nurture your innovation towards commercialisation, get in touch with us here.

